
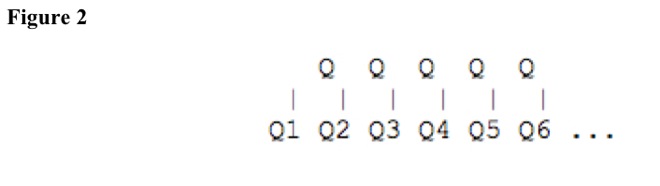

Particles have finite discrete physical-property values {quantum, quantity} {quanta, quantity}. Physical-property values do not vary over a continuous range but have definite values. For example, particle energies have discrete levels and do not have intermediate energies. Discrete physical-property values differ by an amount.
particle properties
Masses are aggregations of particles and so have quanta. As they change speed, masses add or subtract relativistic mass by quanta. Charges are aggregations of electrons and protons and so have quanta. As they change speed, charges add or subtract relativistic charge by quanta. Colors are aggregations of quark colors and so have quanta. As they change speed, colors add or subtract relativistic color by quanta. Strangenesses are aggregations of strangeness and so have quanta. As they change speed, strangenesses add or subtract relativistic strangeness by quanta.
Photons, gluons, bosons, and gravitons (exchange particles) have discrete energies, momenta, and angular momenta (such as spin). Light does not change frequency or wavelength as it travels in vacuum, or as it encounters other electric charges or magnetic fields. Gluons, bosons, and gravitons do not change as they travel or encounter fields. Therefore, forces, energies, and momenta have quanta.
maximum value
Relativity limits values to below maximum, because only infinite energy can make massive particles reach light speed.
Doppler effect
Because light speed is always constant, light sources moving toward or away change light frequency and wavelength. The change occurs at the source, so light does not change frequency and wavelength. as it travels or as it encounters other electric charges or magnetic fields.
Light travels at constant speed. If wavelength decreases, frequency increases. If wavelength increases, frequency decreases. If object is moving away, Doppler effect makes wavelength increase and frequency decrease. If object is moving closer, Doppler effect makes wavelength decrease and frequency increase. Faster motions make greater Doppler effects.
Time dilation is not about Doppler effect, because light is not clock, and light travels at light speed, not lower speed.
ground-state energy
Particle energies have a minimum value (ground state) above zero, because particles have phase-space waves, and waves propagate and so have minimum motion. Particles must move so they cannot have zero energy. Propagating waves have frequency and wavelength. Waves cannot have zero frequency, so waves have a lowest frequency (fundamental frequency) and so lowest possible energy. Electromagnetic-wave energy is frequency times Planck constant.
Because waves have wavelengths, they have uncertain position. Because waves have frequencies, they have uncertain momentum, and uncertain momentum requires minimum energy (uncertainty principle).
energy levels
Waves with the same phase satisfy the Schrödinger wave equation. Therefore, particles can have phase-space waves with harmonic frequencies. Fundamental-frequency harmonics determine allowed energy levels. See Figure 1. Higher frequencies have more energy.
Adjacent wave frequencies differ by fundamental frequency. The energy quantum varies directly with a function of particle phase-space wave fundamental frequency. As frequencies increase, energy differences decrease.
frequency
Wavefunctions with harmonic frequencies solve wave equation. Waves that solve the wave equation resonate in the system, like standing waves that constructively superpose to have net amplitude. Non-standing waves have zero amplitude. Possible standing waves have harmonic frequencies.
quanta
Particle energies, momenta, orbital and spin angular momenta, masses, forces, fields, velocities, accelerations, orbital radii, orbital periods, orbital frequencies, and properties have discrete levels separated by quanta. See Figure 2.
amplitude
Quantum-mechanical waves have amplitude. For any frequency, amplitude relates to probability that particles currently have that wave frequency.
system size
High-energy systems follow quantum mechanics, but phase-space wave wavelengths are too small to detect, so such systems do not appear to have quanta. Physical systems with very small energy or momentum differences, such as subatomic particles, atoms, and molecules, have measurable phase-space wave wavelengths, and such systems require quanta to describe their behavior correctly. See Figure 3. Some quantum-mechanical systems have large space and time differences.

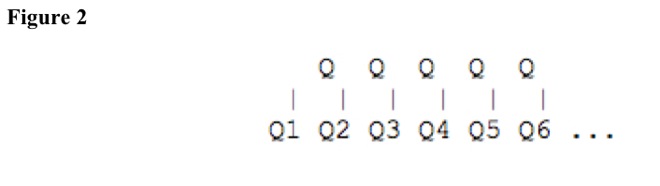

Physical Sciences>Physics>Quantum Mechanics>Quantum
5-Physics-Quantum Mechanics-Quantum
Outline of Knowledge Database Home Page
Description of Outline of Knowledge Database
Date Modified: 2022.0224