In alcohols, amines, or alkyl halides, organic reactions {elimination reaction} can make two substituents leave adjacent carbon atoms and form double bond. Acid or base starts reaction.
acid and alcohol
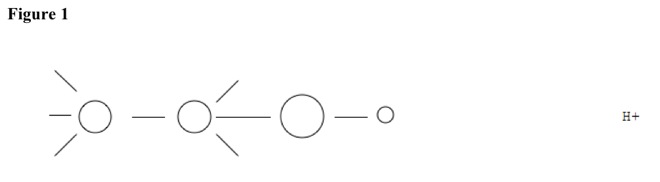
For example, compound can be alcohol. See Figure 1.
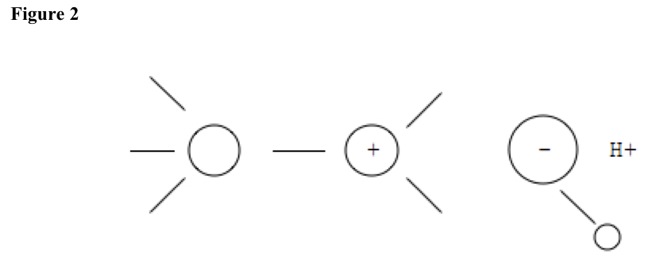
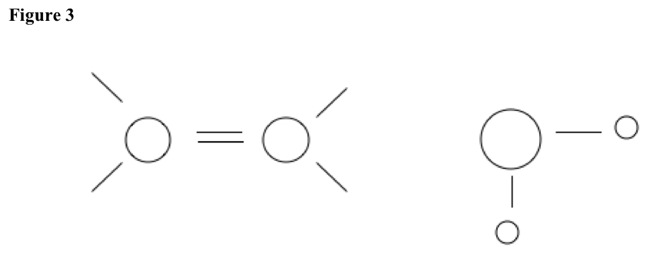
Acid pulls off nucleophile, and hydrogen atom leaves. See Figure 2. Double bond forms, and then acid reforms. See Figure 3.
base and alcohol
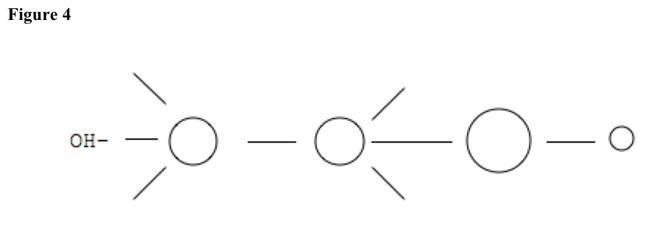
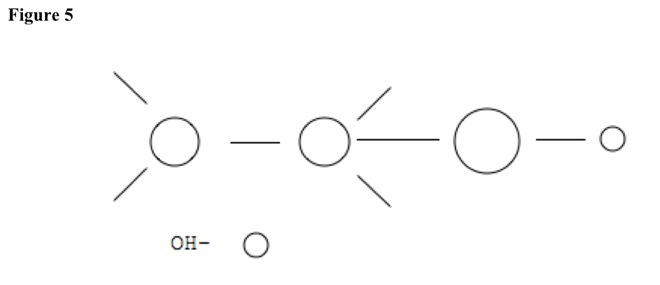
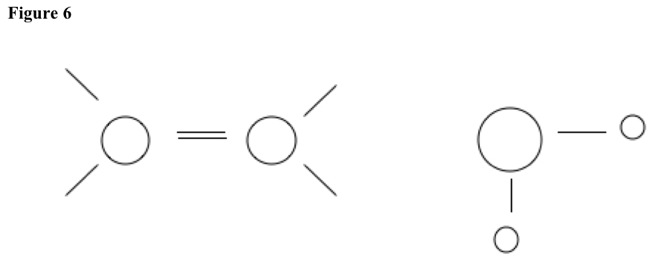
For example, compound can be alcohol. See Figure 4. Base pulls hydrogen atom from carbon with no substituent. See Figure 5. Nucleophile leaves other carbon. Double bond forms, and base reforms. See Figure 6.
E1 elimination
Secondary or tertiary carbon carbocation can form slowly, and then double bond forms quickly {E1 elimination}. Secondary or tertiary carbon is more polar than primary carbon. Acid or base starts this elimination type.
E2 elimination
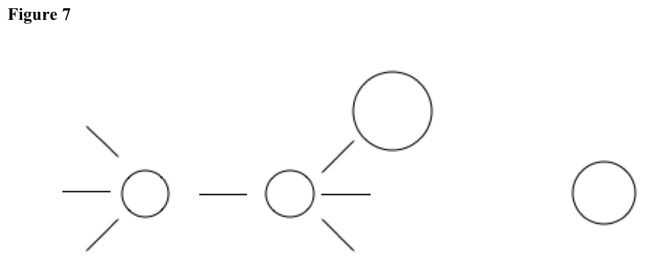
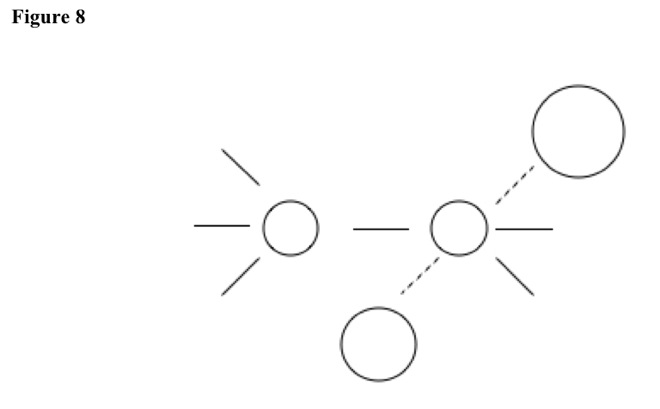
Molecule can slowly push substituent from primary carbon, because primary carbons have no large substituents and low polarization. Bond breaks, and double bond quickly forms {E2 elimination}. See Figure 7, Figure 8, and Figure 9.
base
If strong base is present and reactant is alcohol or alkyl halide, mechanism favors eliminations over substitutions, because base can strongly attract hydrogen atom. If reactant is amine or carboxylic-acid derivative and nucleophile is neutral or acidic, substitution happens more than elimination.









Physical Sciences>Chemistry>Organic>Chemical Reaction
5-Chemistry-Organic-Chemical Reaction
Outline of Knowledge Database Home Page
Description of Outline of Knowledge Database
Date Modified: 2022.0224