Organic reactions {electrophilic addition reaction} can add electrophilic substituent to carbonyl group.
carbonyl
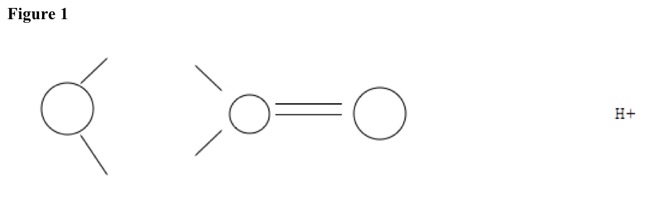
Carbonyl groups have double bond between carbon and oxygen. Carbonyl groups are planar, with two substituents on carbon and none on oxygen. Bond angles are 120 degrees. See Figure 1.
process
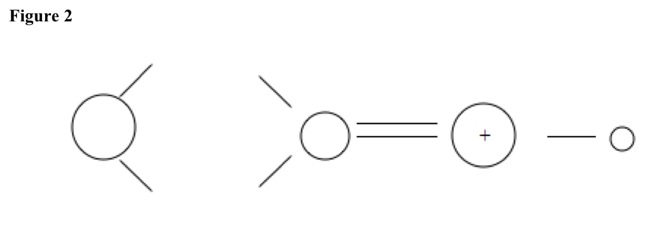
Hydrogen ion attacks carbonyl oxygen and adds to it, making positive charge. Double bond does not break. See Figure 2.
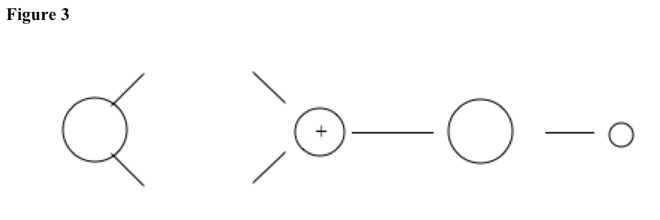
Then positive charge migrates to carbonyl carbon to make carbocation. See Figure 3.
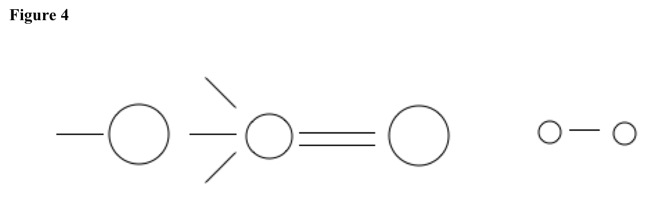
Then water or alcohol adds to carbon. See Figure 4. Hydrogen at carbonyl oxygen and hydrogen at nucleophile leave and combine to make hydrogen gas.




Physical Sciences>Chemistry>Organic>Chemical Reaction
5-Chemistry-Organic-Chemical Reaction
Outline of Knowledge Database Home Page
Description of Outline of Knowledge Database
Date Modified: 2022.0224